MedInsight
Showing 1 to 18 of 41 articles-

Reusable Pregnancy Tests: Eco-Friendly, Cost-Effective & Accurate Solutions for Modern Pregnancy Testing
Enter the eco-friendly solution: reusable pregnancy tests. These sustainable, innovative devices are not only shaping the way we test for pregnancy but are also harmonizing with the increasing global emphasis on environmental consciousness. -

New update to Candidate List of Substances of Very High Concern (SVHCs)
On 7th November 2024, the ECHA (European Chemicals Agency) released the new Candidate List of SVHCs with the addition of one new substance. The current list of SVHCs now contains 242 substances. -

Emerging Solutions to Address The Environmental Impact of Pregnancy Tests
Disposable digital pregnancy tests add to the plastic waste crisis through e-waste, non-recyclable materials, and toxic batteries. Emerging sustainable options like reusable and biodegradable tests offer greener, eco-friendly alternatives to reduce this environmental impact. -

The IVDR extension amendment bill was officially published in the EU Official Journal
The IVDR transition period has been extended, allowing importers to continue selling products certified under the previous IVDD directive. This extension provides more time to comply with labeling, documentation, and certification requirements, minimizing non-compliance risks and maintaining market access.
-

Technological Innovation in the Medical Device Industry: A 2024 Overview
Discover how AI, wearables, and cutting-edge diagnostics are driving innovation in the medical device industry in 2024, reshaping healthcare delivery while navigating regulatory and cybersecurity challenges. -

EU releases Q&A document on IVDR transition period extension and MDR transition period extension Q&A document update
The European Commission has released a Q&A document regarding the extension of the IVDR transition period, as well as an updated Q&A document on the extension of the MDR transition period, pursuant to Regulation 2024/1860, which was recently published. -

Surging Demand for Reusable Digital Ovulation Tests: Accuracy Meets Convenience
The market demand for reusable digital ovulation tests is on the rise, driven by growing consumer awareness around fertility tracking, interest in sustainable health products, and advancements in digital health technologies. -

The application value of AI technology to family diagnosis
The application value of AI technology in family diagnosis is great, making diagnosis more accurate, convenient and efficient. By providing personalized health management solutions and empowering users with more health knowledge, AI is changing the way individuals approach health, leading to better health outcomes and more efficient healthcare systems. -

FDA and EU expand Mutual Recognition Agreement, again!
In 2017, the FDA initiated MRAs with EU and UK, for human drugs. On May 30th, the FDA once again signed an MRA, with 16 EU countries, this time concerning veterinary drugs. -
Keeping Tabs on Your Kidneys: The Importance of Using a Renal Function Meter
Discovering the significance of maintaining optimal kidney health is crucial for overall well-being. And now, keeping tabs on your kidneys has never been easier with the introduction of the Renal Function Meter. -

Home Healthcare with Hemoglobin Meters
Before exploring their practical uses, it's essential to grasp the fundamental concept of hemoglobin meters.Hemoglobin meters are compact medical instruments meticulously engineered for gauging the hemoglobin concentration in an individual's blood. -
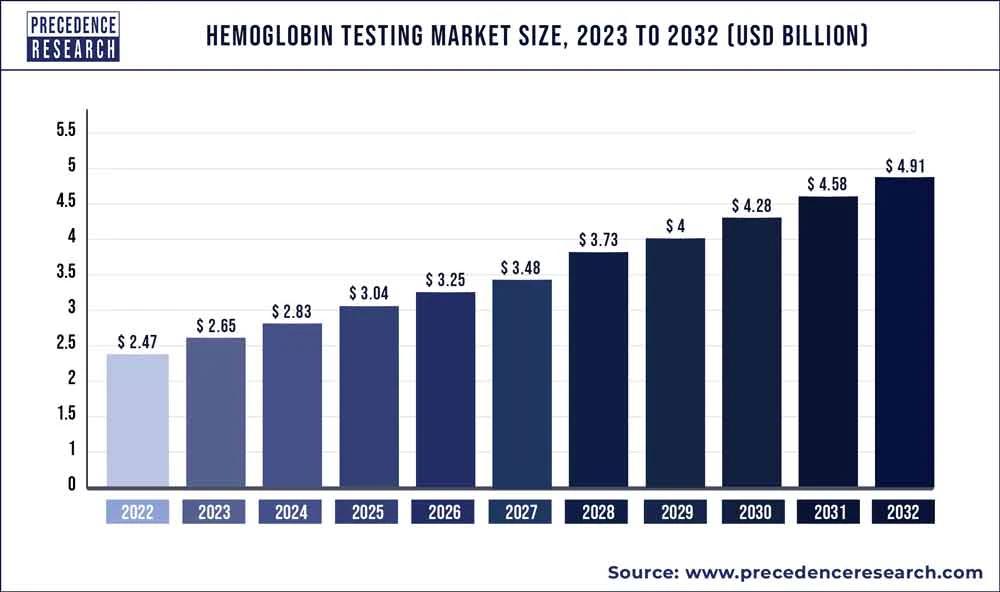
Promising Outlook: Portable Hemoglobin Meter Market
Before delving into the portable hemoglobin meter market, it's important to understand the significance of hemoglobin in healthcare. -

European Cholesterol Testing Landscape
Low-density lipoprotein (LDL), commonly referred to as "bad" cholesterol, is recognized for its role in heightening the vulnerability to heart disease and strokes. -
Cholesterol Testing Market Analysis
The global cholesterol testing products market achieved a substantial milestone, surging to a value of US$ 8.5 billion in 2022. Anticipating a trajectory of remarkable expansion, the market is poised to ascend to even greater heights, potentially reaching a staggering US$ 15.9 billion by the year 2030. -
Easier Parenthood: Wee-Enhanced Digital Pregnancy Tests
Embarking on the journey of parenthood is an exhilarating and life-altering experience, filled with anticipation, wonder, and a touch of nervousness. Enter the digital pregnancy test with weeks estimator, an innovation designed to offer expectant parents peace of mind during this pivotal time. -
Impact of European AI Act on Medical Devices
EU AI Act is to be the world's first AI law. European Parliament passed the draft on June 14. However, it faced opposition from industry organizations like MedTech Europe, the Confederation of European Business, and Team-NB (European Association for Medical Devices Notified Bodies). -

Decoding QMSR: FDA QMSR & ISO 13485 Harmony
QMSR will align FDA's medical device quality management system with ISO 13485:2016. It will be released by the end of this year and become effective one year later. This harmonization is expected to save medical device companies at least $439 million over the next decade. -

Advantages: Digital Pregnancy Tests with Weeks Indicator
Embracing digital innovation, these tests eliminate the guesswork and provide specific information, empowering you with the knowledge you need as you embark on this incredible journey.
- Subscribe MedInsight
- Subscribe MedInsight
- Subscribe MedInsight
- Subscribe MedInsight
- Subscribe MedInsight



















