FDA and EU expand Mutual Recognition Agreement, again!

On May 31, 2023, the U.S. Food and Drug Administration (FDA) and the European Union (EU) have announced the expansion of the U.S.-EU Mutual Recognition Agreement (MRA) Sectoral Annex for Pharmaceutical Good Manufacturing Practices (GMP). This expansion now includes inspections of veterinary pharmaceuticals, also known as "animal drugs."
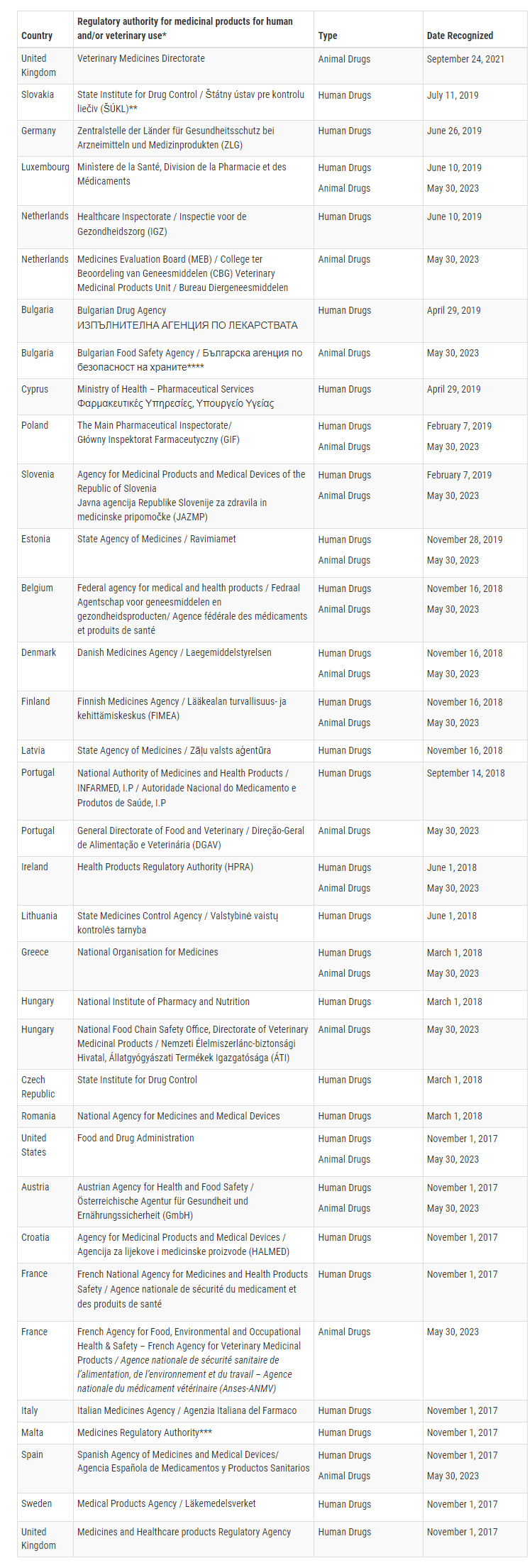
Currently, the FDA has confirmed that 16 European Union Member States are capable of conducting GMP inspections for specific veterinary products at a level equivalent to the United States. These Member States are Austria, Belgium, Bulgaria, Denmark, Estonia, Finland, France, Greece, Hungary, Ireland, Luxembourg, Netherlands, Poland, Portugal, Slovenia, and Spain.
With this expanded MRA, the FDA can rely on GMP inspections conducted by these 16 Member States for veterinary product facilities, while the European Union will rely on inspections conducted by the FDA. This mutual recognition enhances regulatory cooperation and streamlines the oversight of veterinary pharmaceuticals between the FDA and the EU.
What's MRA?
Mutual Recognition Agreements (MRAs) facilitate collaboration between the FDA and foreign regulatory authorities, allowing drug inspectors to rely on inspection information conducted in each other's jurisdictions. The Food and Drug Administration Safety and Innovation Act, implemented in 2012, grants the FDA the authority to establish agreements recognizing drug inspections conducted by foreign regulatory authorities that meet U.S. requirements.
MRAs serve two key purposes:
1. Enhancing efficiencies: By avoiding duplicate inspections, MRAs promote streamlined processes for both U.S. and foreign regulatory systems.
2. Resource allocation: MRAs enable the reallocation of resources to focus on inspecting drug manufacturing facilities with potentially higher public health risks worldwide.
Who has signed MRAs with the FDA?
Currently, the FDA has MRAs in place with the European Union (EU) and the United Kingdom (UK). Additionally, on January 12, 2023, the FDA signed an MRA with Switzerland. However, before this MRA takes effect, the FDA must assess Switzerland's inspection capabilities to ensure they meet U.S. requirements. Similarly, the Swiss Agency for Therapeutic Products (Swissmedic) must evaluate the FDA's compliance with Swiss requirements.

Limitations
The capability assessments currently pertain to routine surveillance inspections. However, there is potential for the following product and inspection types to be included in the agreement's coverage after further evaluation:
1. Vaccines for human use
2. Plasma-derived pharmaceuticals
3. Investigational products (clinical trial material), specific to each agreement
The FDA and the UK have discussed the possibility of expanding the MRA's scope to include vaccines and plasma-derived pharmaceuticals for human use. A decision on this matter will be revisited in July 2025 after conducting additional assessments.
Similarly, the FDA and the EU have also explored expanding the MRA to include vaccines and plasma-derived pharmaceuticals for human use. The FDA will re-evaluate this issue in July 2025, considering the findings from further assessments.
Excluded from the MRA scope are: Advanced Therapy Medicinal Products (ATMPs), human blood, human plasma, human tissues and organs, cells (Swissmedic MRA), and veterinary immunologicals.
PS: If you want our team to customize a new homecare product, with FDA, MDR, ISO, FSC and more, please contact insights@medasiagroup.com
WE RECOMMEND
Related posts
- Subscribe MedInsights
- Subscribe MedInsights
- Subscribe MedInsights
- Subscribe MedInsights
- Subscribe MedInsights